A surge in counterfeit Botox procedures is putting lives at risk, with two Louisiana residents already hospitalized due to unlicensed injections.
Story Highlights
- Two Louisiana residents hospitalized after counterfeit Botox injections.
- LDH issues a warning about unlicensed cosmetic procedures.
- Counterfeit Botox is part of a growing national concern.
- Online platforms are facilitating the sale of counterfeit products.
- Regulatory agencies call for increased vigilance and reporting.
Counterfeit Botox Poses Severe Health Risks
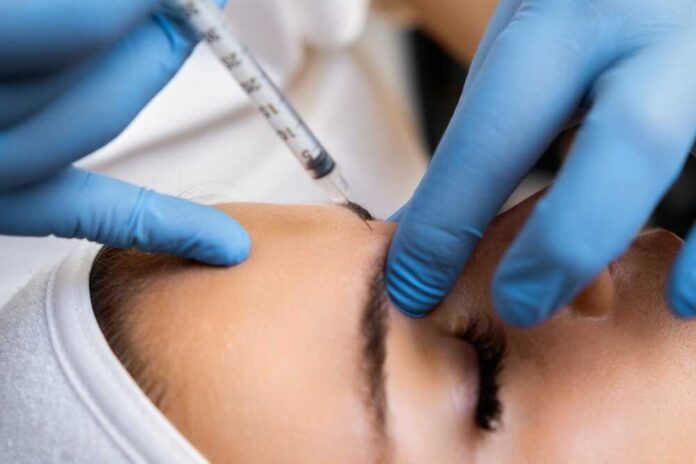
In September 2025, the Louisiana Department of Health (LDH) reported two alarming cases of severe illness. These incidents involved residents who received counterfeit or unlicensed Botox injections and subsequently developed botulism-like symptoms. The LDH issued a public warning, urging residents to avoid unlicensed cosmetic procedures, which have become a growing national concern. This development highlights the dangers posed by counterfeit Botox, often facilitated through online platforms.
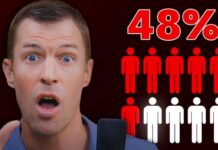
Counterfeit Botox poses a significant risk due to its potential for contamination, improper storage, or dangerous concentrations. These factors can lead to severe health outcomes, including botulism. The FDA and CDC have previously warned about these dangers, emphasizing the importance of receiving injections only from licensed providers. The Louisiana cases add to a disturbing trend, with at least 22 people hospitalized across 11 states since 2023 due to counterfeit Botox complications.
Watch:
The Role of Online Platforms
Online platforms play a critical role in the distribution of counterfeit Botox. These platforms bypass regulatory oversight, allowing unlicensed providers to sell dangerous products. This trend complicates enforcement efforts by regulatory agencies like the FDA, which struggles to police the online marketplace effectively. There is an urgent need for enhanced monitoring and enforcement to prevent further incidents and protect public health.
Patients seeking cosmetic enhancements may be unaware of the risks associated with unlicensed procedures. Driven by the appeal of lower costs or convenience, they often fall victim to these dangerous practices. As the black market for cosmetic products grows, public education becomes essential to inform consumers about the potential hazards and encourage vigilance.
Regulatory and Public Health Responses
The LDH and other agencies are stepping up efforts to combat the dangers of counterfeit Botox. They have issued public warnings and established hotlines for reporting suspicious activities. Regulatory bodies are also working to increase scrutiny on online sales of cosmetic products, potentially leading to stricter regulations in the future. These measures aim to prevent further health crises and restore public confidence in legitimate cosmetic procedures.
The cosmetic and medical aesthetics industry may face increased regulation and oversight due to this growing concern. The pressure on online platforms to enhance monitoring and enforcement against illegal sales is intensifying. This situation underscores the need for collaboration between regulators, law enforcement, and social media companies to address the root causes of the problem effectively.
Sources:
Two Louisiana residents report severe illness following counterfeit Botox injection, LDH says
Louisiana Botox counterfeit cases
Louisiana Botox warning, hospitalizations, and counterfeit
Louisiana botulism officials warn