Scientists have discovered that “poo pills” containing transplanted gut bacteria from healthy donors may be our best weapon against antibiotic-resistant superbugs, potentially saving millions of lives worldwide.
At a Glance
- Fecal microbiota transplants (FMT) in pill form show promise in eliminating antibiotic-resistant bacteria from the gut
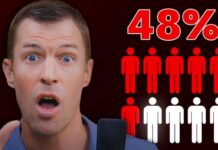
- In one small trial, 8 out of 9 participants tested negative for antibiotic-resistant bacteria after 36 days of treatment
- FMT has already shown a 90% success rate in treating C. difficile infections
- Antimicrobial resistance is rapidly increasing and could become the leading cause of death globally by 2050
- Over 450 microbiome-based medicines are in development, potentially replacing traditional antibiotics
The Growing Superbug Crisis
Antimicrobial resistance (AMR) is rapidly becoming one of the most significant health threats of our time. Drug-resistant infections killed nearly 5 million people in 2019 alone, exceeding deaths from both HIV and malaria. Experts warn that without effective intervention, AMR could become the world’s leading cause of death by 2050. The problem is particularly acute in regions with poor water quality, sanitation, and limited access to proper diagnostics, where resistant strains spread more easily through communities and healthcare facilities.
Former UK Chief Medical Officer Sally Davies has warned that “antimicrobial resistance could kill us before the climate crisis does,” highlighting the urgency of finding alternative treatments. Poor prescribing practices, overuse of antibiotics in agriculture, and the natural evolution of bacteria have accelerated this crisis, leaving doctors with fewer options to treat even common infections. As traditional antibiotics lose effectiveness, researchers have turned to novel approaches – including looking to human waste for answers.
How Fecal Transplants Work
Fecal microbiota transplants involve transferring beneficial gut bacteria from a healthy donor to a patient, effectively repopulating the intestinal tract with microorganisms that can outcompete harmful pathogens. While initially the procedure sounds unpleasant, scientists have developed a way to package the treatment in pill form, making it more acceptable to patients. These “poo pills” contain freeze-dried fecal matter from carefully screened donors who meet strict health criteria.
The treatment first gained recognition for its effectiveness against Clostridioides difficile (C. diff) infections, a dangerous intestinal condition that often occurs after antibiotic use disrupts the natural gut flora. FMT has shown approximately 90% success in treating recurrent C. diff infections, significantly outperforming traditional antibiotic approaches. This success has prompted researchers to investigate whether similar principles could apply to combating other antibiotic-resistant bacteria.
Promising Clinical Results
Recent trials testing fecal transplants against antibiotic-resistant bacteria have yielded encouraging results. In one small clinical study, researchers administered FMT to patients colonized with antibiotic-resistant organisms. After 36 days, 8 out of 9 participants tested negative for these dangerous bacteria. Unlike many previous approaches that only temporarily suppress antibiotic-resistant bacteria, FMT appears to effectively eliminate them by restoring a healthy balance of gut microbes.
UK doctors have also begun trialing “poo pills” to combat superbug infections in hospitalized patients. Their approach aims to replace antibiotic-resistant bacteria in the bowel with healthy gut microbes. Early results indicate that patients are willing to take the treatment, and importantly, the donor bacteria successfully establish themselves in the recipient’s gut for at least a month, suggesting long-term effectiveness may be possible.
The Future of Microbiome Medicine
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) reports that over 450 microbiome-based medicines are currently in development, signaling a potential paradigm shift in how we treat infections. Rather than relying solely on antibiotics that kill bacteria indiscriminately, these approaches work with the body’s natural systems to restore healthy bacterial communities. Researchers are also developing synthetic alternatives that contain only the beneficial bacteria from feces, making treatments more standardized and potentially more palatable to patients.
While fecal transplants show tremendous promise, they represent just one approach in the fight against superbugs. Other innovative strategies include using AI to discover new antibiotics (like the recently identified abaucin), developing bacteriophage therapies that use viruses to target specific bacteria, and increasing vaccination efforts to prevent infections before they require treatment. Together, these approaches may help humanity regain ground in the ongoing battle against antibiotic resistance.