Revolutionary blood biomarkers are transforming early Alzheimer’s detection, offering new hope for millions while changing how we approach neurodegenerative diseases.
At a Glance
- New blood tests can detect Alzheimer’s disease biomarkers years before symptoms appear, with up to 92% accuracy in some cases
- Key biomarkers include p-tau217, p-tau181, NfL, and GFAP, which signal neurodegenerative changes
- Multiple blood tests have received FDA Breakthrough Device Designation, accelerating their path to clinical use
- These tests could make screening more accessible and affordable compared to current diagnostic methods
- Pairing different biomarkers improves predictive accuracy, potentially revolutionizing early intervention strategies
The Promise of Blood-Based Biomarkers
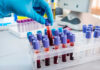
The landscape of Alzheimer’s disease diagnosis is undergoing a profound transformation with the emergence of blood-based biomarkers. These tests measure specific proteins circulating in the bloodstream that are linked to the disease process, including beta-amyloid and phosphorylated tau (p-tau) proteins. Unlike traditional diagnostic methods that require expensive brain imaging or invasive spinal taps, these blood tests offer a simpler, more accessible approach that could revolutionize early detection efforts. The PrecivityAD2 test, one of several emerging options, has demonstrated remarkable accuracy, predicting Alzheimer’s in 88% to 92% of cases.
Early diagnosis remains crucial in addressing Alzheimer’s disease, as treatment is most effective before irreversible brain damage or cognitive decline occurs. Blood tests can potentially identify disease markers years or even decades before symptoms manifest, creating a wider window for therapeutic intervention. This represents a significant advancement in our ability to address a condition that affects millions of older adults worldwide and has historically been difficult to diagnose definitively without extensive, expensive testing.
A breakthrough in the diagnosis of Alzheimer's disease is the p-tau217 blood test. But there's mystery and controversy surrounding it.https://t.co/4TxfHXbRaP @Nature feature @alison_c_abbott pic.twitter.com/lVkBglkzJx
— Eric Topol (@EricTopol) August 7, 2024
Breakthrough Technologies Gaining Recognition
Several prominent blood tests for Alzheimer’s disease have recently received FDA Breakthrough Device Designation, highlighting their potential clinical impact. Roche’s Elecsys® pTau217 plasma biomarker test targets amyloid pathology to support earlier diagnosis, while Quanterix’s Simoa® phospho-Tau 217 blood test offers similar capabilities. These designations accelerate the regulatory pathway and underscore the significance of these technologies in addressing an urgent medical need. The pTau217 biomarker, specifically, has shown remarkable ability to distinguish Alzheimer’s from other neurodegenerative disorders.
Access to these innovative tests varies considerably. Some require a physician’s order, while others are available directly to consumers. Insurance coverage remains limited, meaning patients often bear the full cost. However, even with these limitations, blood tests represent a significant advance in accessibility compared to traditional diagnostic methods like positron emission tomography (PET) scans or lumbar punctures to collect cerebrospinal fluid. As these technologies mature and gain wider acceptance, their integration into routine clinical care could fundamentally change how Alzheimer’s is diagnosed and managed.
📣#BrainMatters webinar this Wed., 12/4, 7-8pm🧠
"#Biomarkers, #Blood #Tests & #Breakthroughs: The Importance of #Early #Testing & #Diagnosis for #Dementia"
Speakers:
👉Dr. Snyder (Alzheimer's Association) &
👉Dr. Moghekar (JH Alzheimer's Disease Research Center)Free… pic.twitter.com/yPk7OAIXBs
— Johns Hopkins Memory & Aging (@JH_Memory_Aging) December 2, 2024
Advancing Predictive Capabilities Through Research
Recent research has demonstrated the impressive predictive power of blood biomarkers for Alzheimer’s risk assessment in community settings. A groundbreaking study published in Inside Precision Medicine found that elevated baseline levels of p-tau181, p-tau217, neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP) were observed in participants who later developed dementia. These findings suggest that blood tests could serve as effective screening tools years before clinical symptoms emerge, potentially transforming prevention strategies for high-risk individuals.
Researchers have discovered that combining multiple biomarkers significantly improves predictive accuracy. The pairing of p-tau217 and NfL demonstrated the strongest performance for predicting all-cause dementia. Machine learning techniques, including Support Vector Machine (SVM) and Random Forest algorithms, are enhancing analytical capabilities by identifying patterns among complex biomarker data. These approaches show promise for distinguishing between cognitively normal individuals and those with mild cognitive impairment or Alzheimer’s disease, potentially allowing for more personalized risk assessments and treatment approaches.
Future Directions and Clinical Applications
For blood biomarkers to reach their full potential in clinical practice, integration with other diagnostic tools will be essential. Researchers suggest combining blood tests with clinical assessments, genetic markers, and cognitive testing to create comprehensive diagnostic protocols. Sensitivity analyses have shown stronger associations between blood biomarkers and Alzheimer’s risk in specific populations, including younger individuals, women, and those carrying the APOE ε4 genetic risk allele. This points to opportunities for tailoring diagnostic approaches to different demographic groups.
As these blood tests become more widely available, education for healthcare providers and patients will be crucial. Understanding the implications of test results, appropriate follow-up care, and the limitations of current diagnostic capabilities will help manage expectations. While blood biomarkers represent a major advancement, they are one component of a comprehensive approach to Alzheimer’s diagnosis and management. Their greatest value lies in their potential to identify at-risk individuals early, when interventions might have the greatest impact on disease progression and quality of life.
Sources:
https://www.alz.org/alzheimers-dementia/research-and-progress/earlier-diagnosis
https://www.medtechdive.com/news/roche-eli-lilly-fda-breakthrough-alzheimers-blood-test/713011/