FDA experts sound alarm over widespread use of unapproved dermal fillers in the décolletage area.
Story Highlights
- FDA panel demands more data collection before approving décolletage fillers due to serious safety concerns
- Off-label filler use surges without proper regulatory oversight or adverse event tracking
- Experts warn of breast imaging interference and inability to remove certain filler types
- High-risk populations including pregnant and breastfeeding women urged to avoid procedures
Regulatory Oversight Finally Addresses Safety Gaps
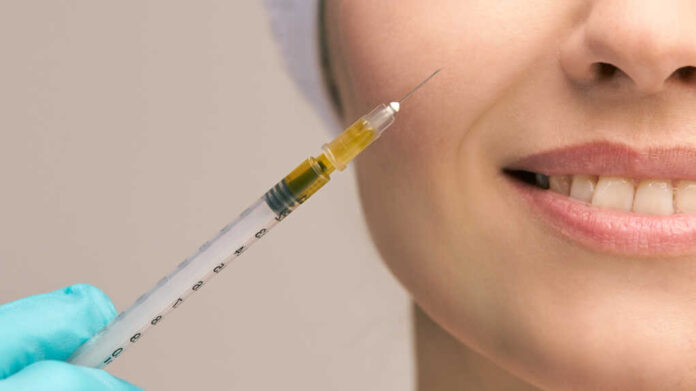
The FDA’s General and Plastic Surgery Devices Panel convened on August 13, 2025, to address mounting concerns about dermal filler use in the décolletage area. This triangular region from the neck to between the breasts has seen explosive growth in off-label procedures despite lacking FDA approval. The panel’s recommendations highlight a troubling pattern of cosmetic procedures outpacing regulatory safeguards, leaving patients vulnerable to poorly understood risks.
Watch:
Critical Safety Concerns Emerge from Expert Analysis
Panel experts identified multiple serious risks unique to décolletage filler injections. The area’s proximity to breast tissue raises concerns about interference with mammograms and other breast cancer screening methods. Certain filler types cannot be removed once injected, creating permanent changes that could complicate medical imaging for years. These concerns are particularly troubling given the FDA’s acknowledgment that adverse events are significantly underreported in this largely unregulated space.
Off-Label Use Expands Despite Regulatory Gaps
The surge in décolletage filler procedures reflects broader trends in cosmetic medicine, driven partly by social media influence and patients seeking procedures after weight loss. However, many treatments occur in non-medical settings where proper data collection and adverse event reporting are virtually non-existent. This regulatory blind spot undermines patient safety and prevents authorities from understanding the true scope of complications and risks associated with these procedures.
Industry Accountability and Patient Protection Measures
Professional societies including the American Academy of Dermatology and American Society of Plastic Surgeons are developing safety guidelines while manufacturers like Merz Aesthetics seek formal FDA approval. The panel specifically recommended excluding high-risk populations such as pregnant women, those with darker skin types, and patients with history of breast cancer treatment. These measures represent a long-overdue focus on patient safety over cosmetic industry profits and expansion.
The FDA’s intervention signals growing recognition that the cosmetic industry has operated with insufficient oversight for too long. While manufacturers claim low adverse event rates, the lack of comprehensive data collection makes such assertions meaningless. This case demonstrates the importance of regulatory agencies fulfilling their protective role rather than allowing industries to self-regulate at patients’ expense.
Sources:
Updated Meeting Time and Public Participation Information – August 13, 2025 General and Plastic Surgery Devices Panel
FDA Advisory Committee Meeting Document – Patient Selection and Risk Factors
FDA Executive Summary – Dermal Fillers in Décolletage Area Safety Review
FDA Panel Meeting Materials – Clinical Data and Regulatory Considerations
Federal Register Notice – General and Plastic Surgery Devices Panel Meeting