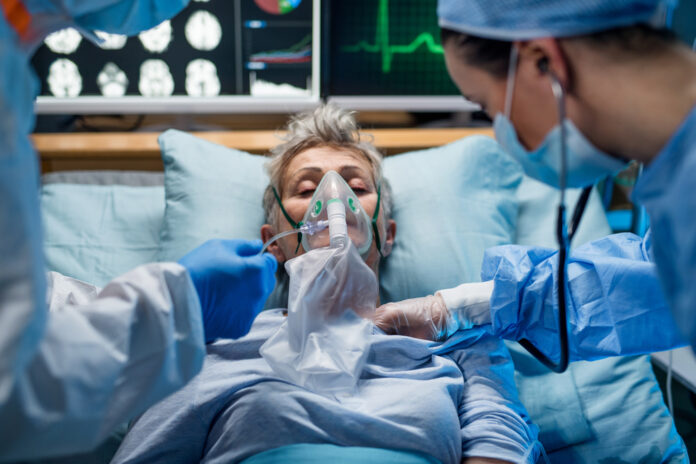
Two years ago, researchers at Veru began testing Sabizabulin on patients with COVID-19. Scientists suspected that Sabizabulin, which blocks cells from building microtubules, might reduce or prevent the virus from replication. Over the course of two years, they worked with volunteers hospitalized with COVID to assess the effect the medication might have on their treatment.
In April, the test was stopped early as the results toward positive use did not support continuing to offer a placebo.
What Are Microtubules?
Microtubules are essentially tubes that carry material from one part of the cell to another part. Sabizabulin was originally developed at the University of Tennessee to fight cancer. The reasoning is that fast-growing tumor cells depend on microtubules for their rapid growth.
If the path to building microtubules is blocked, the cell’s growth might be stopped or at least slowed down. The research has many possible uses, even beyond cancer and COVID.
Recently, Veru concluded its testing with results that seem to be very positive. Researchers agree that more testing is needed, as the sample group was small, but adding another medication to the fight against COVID is exciting news.
What Medications Are Available for In-Hospital Patients With COVID?
The addition of Sabizabulin to the arsenal against COVID would be beneficial as medications to assist in the fight are limited. Other medications that have been used with some success include the following:
Dexamethasone: Dexamethasone is a steroid medication that has been effective in reducing deaths by one-third. In testing Sabizabulin, patients receiving dexamethasone were allowed to continue those treatments.
Baricitinib: This arthritis drug has also been studied to assess its impact on COVID patients in the hospital.
Tocilizumab: Approved for emergency use in 2021, Tocilizumab has also been beneficial for patients hospitalized with COVID. The medication, typically used to assist with inflammatory diseases, works to decrease inflammation.
These medications were all shown to have positive effects on hospitalized patients with COVID. It’s important to understand that with all four medications, patients who received the treatments were either on a ventilator or were receiving oxygen.
What Are the Next Steps?
At this point, Veru has applied to the Food and Drug Administration for emergency approval of Sabizabulin. If approved, along with the two mentioned above, a better outcome for COVID19 patients who are hospitalized may be on the way. With the rise and fall of cases, along with new variants, increased means to fight back are needed.